肺診斷-固態腫瘤DNA/RNA檢測
《非小細胞肺癌NCCN指南》明確指出,驅動基因突變狀態是靶向藥物治療的重要療效預測因素, 人類EGFR、HER2、KRAS、BRAF基因突變,ALK、ROS1、RET、NTRK1、NTRK2、NTRK3基因融合和MET外顯子14跳躍突變患者可以從相應酪氨酸激酶抑制劑治療中獲益,在進行標靶治療前需要對基因的突變狀態進行檢測,並強烈建議進行更廣泛的有效基因的狀態檢測,因此對NSCLC患者的多基因突變聯合檢測可為患者提供更精準的治療。
NCCN 指引建議標靶用藥
ALK Rearrangement
- First-line therapy
- Alectinib
- Brigatinib
- Ceritinib
- Crizotinib
- Lorlatinib
- Subsequent therapy
- Alectinib
- Brigatinib
- Ceritinib
- Lorlatinib
BRAF V600E Mutation
- First-line therapy
- Dabrafenib/trametinib
- Dabrafenib
- Vemurafenib
- Subsequent therapy
- Dabrafenib / trametinib
EGFR Exon 19 Deletion or L858R
- First-line therapy
- Afatinib
- Erlotinib
- Dacomitinib
- Gefitinib
- Osimertinib
- Erlotinib + ramucirumab
- Erlotinib + bevacizumab
- (nonsquamous)
- Subsequent therapy
- Osimertinib
EGFR S768I, L861Q, and/or G719X
- First-line therapy
- Afatinib
- Erlotinib
- Dacomitinib
- Gefitinib
- Osimertinib
- Subsequent therapy
- Osimertinib
EGFR Exon 20 Insertion Mutation
- Subsequent therapy
- Amivantamab-vmjw
- Mobocertinib
ERBB2 (HER2) Mutation
- Subsequent therapy
- Fam-trastuzumab
- deruxtecan-nxki
- Ado-trastuzumab emtansine
KRAS G12C Mutation
- Subsequent therapy
- Sotorasib
MET Exon 14 Skipping Mutation
- First-line / Subsequent therapy
- Capmatinib
- Crizotinib
- Tepotinib
NTRK1/2/3 Gene Fusion
- First-line/Subsequent therapy
- Larotrectinib
- Entrectinib
RET Rearrangement
- First-line/Subsequent therapy
- Selpercatinib
- Pralsetinib
- Cabozantinib
ROS1 Rearrangement
- First-line therapy
- Ceritinib
- Crizotinib
- Entrectinib
- Subsequent therapy
- Lorlatinib
- Entrectinib
Gene List
含括上述所有NCCN指引建議標靶用藥所對應的基因變異
| DNA | RNA |
|---|---|
|
|
註:DNA檢測SNV/InDel
註:RNA檢測Fusion/Skipping
檢測對象:
肺癌癌症患者
初次診斷為晚期癌症患者
使用標準治療成效不佳者
檢體種類:
腫瘤組織切片檢體
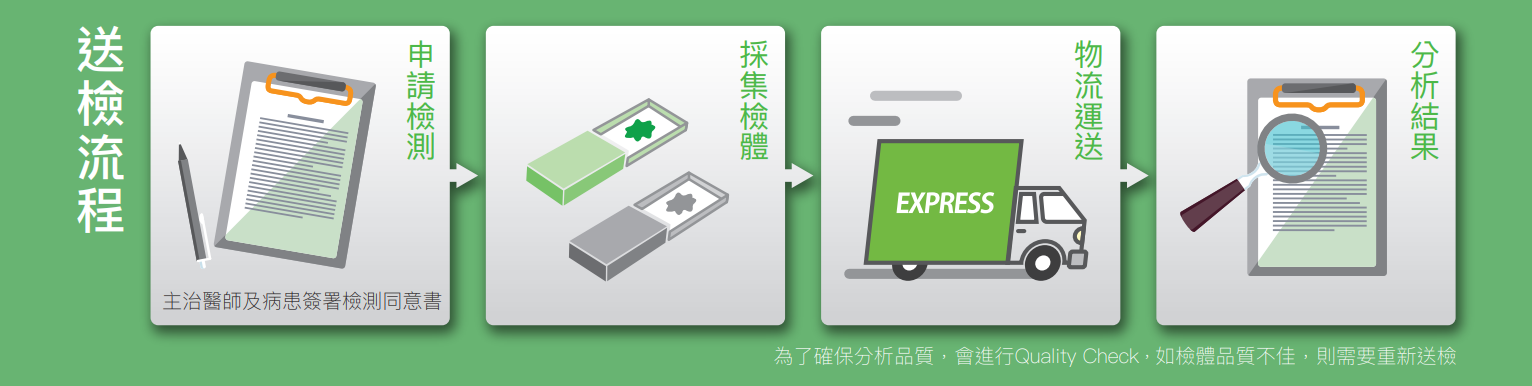
檢測流程
資料下載

